Abstract
Background The recent phase 3 ADMIRAL trial has shown that outcomes of relapsed/refractory (R/R) FLT3-mutated acute myeloid leukemia (AML) patients were improved by the single-agent FLT3 tyrosine kinase inhibitor gilteritinib as compared with salvage chemotherapy. In this trial, overall survival (OS) was significantly improved in the gilteritinib arm and complete remission (CR/CRi), after excluding those obtained after allogenic hematopoietic stem cell transplantation (Allo-HSCT) were 26.3% in the gilteritinib group.
Methods Here, the real-world efficacy and safety of gilteritinib was assessed in an ambispective study that included 167 R/R FLT3-mutated AML patients (Cohort A) from the French early access program ELEGANCE between March 1st, 2019, and March 1st, 2021. Gilteritinib was administered to 27 patients in association with other treatments, while 140 received it as single agent (Cohort B), including 67 previously treated by intensive chemotherapy and midostaurin (Cohort C).
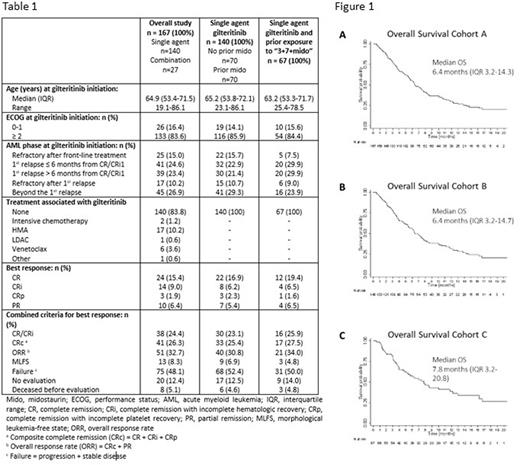
Results Characteristics of the three cohorts at gilteritinib initiation are described in Table 1. Briefly, in the whole cohort A, there were 141 (84.4%) cases of de novo AML, FLT3 mutation was ITD in 104 (62.3%) and TKD in 33 (19.8%), whereas 36 (21.6%) patients were FLT3 wild-type at diagnosis, acquired mutation later in disease evolution and were FLT3 mutated at gilteritinib initiation. Finally, 6 (3.6%) patients had both FLT3-ITD and TKD mutations. The median FLT3-ITD/wt ratio was 0.50 (IQR, 0.12-0.70 and range 0.01-1.90), and the median size of ITD was 48 bp (IQR, 30-75, range 3-408). Most patients (136, 81.4%) had an intermediate cytogenetic risk, and 84 (50.3%) had an NPM1 co-mutation. The main differences in patient characteristics in this study, compared to ADMIRAL, were ECOG ≥ 2 (83.6% vs 16.6%), FLT3-TKD mutations (21.0% vs 8.5%), primary induction failure (15.0% vs 40.0%) and previous lines of treatment (beyond 2nd in 37.1% vs 0.0%).
The rates of response to gilteritinib are described in Table 1. Moreover, CRc (CR + CR without hematological recovery + CR with incomplete platelet recovery) to gilteritinib as single agent in subgroups defined as per AML phase at gilteritinib initiation were 4.5% (N=22) for patients refractory after front-line, 37.5% (N=32) for those relapsing less than 6 months from CR/CRi, 26.7% (N=30) when relapse occurred after 6 months and 16.1% (N=56) for patients refractory after 1st relapse and beyond. Finally, 32 (19.2%), 25 (17.9%) and 15 (22.4%) patients received an Allo-HSCT after gilteritinib, in CRc for 21 (65.6%), 17 (68.0%) and 11 (73.3%) patients in cohorts A, B and C, respectively. In terms of safety, in the subgroup of patients treated with gilteritinib as single agent (Cohort B), 39 (28.3%) experienced a serious adverse event (SAE) during gilteritinib treatment: infection in 22 (56.4%), hemorrhage in 1 (2.6%), cardiovascular in 4 (10.3%), hepatic in 4 (10.3%), differentiation syndrome in 2 (5.2%) and other SAE in 6 (15.4%). In terms of health care resource consumption, the median duration of hospitalization during the first 6 months of treatment was 9.0 days (IQR 0-29, range 0-117) with a median of 7 RBC units (IQR 0-14, range 0-54) and of 5 platelet units (IQR 0-13.5, range 0-56) transfusions.
After a median follow-up of 14.5 months, median OS and 12-month OS were 6.4 months (IQR, 3.2-14.7) and 31.4% (95%CI 22.4-39.7) in the 140 patients who received gilteritinib as single agent, and 7.8 months (IQR, 3.2-20.8) and 38.3% (95%CI 26.0-50.4) in the subgroup of 67 patients previously treated by intensive chemotherapy and midostaurin (Figure 1). Multivariate analyses disclosed female gender (HR 1.61, 95%CI 1.07-2.42, p=0.02), adverse cytogenetic risk (HR 2.52, 95%CI 1.24-5.13, p=0.01) and allo-HSCT after gilteritinib (HR 0.13, 95%CI 0.05-0.37, p<0.0001) as factors significantly and independently associated with OS.
Conclusion Although patients in this study were more heavily pretreated, these real-world data reproduce ADMIRAL results and provide new insights in the outcome of patients previously treated by intensive chemotherapy and midostaurin and beyond the 2nd line of treatment.
Disclosures
Dumas:Janssen: Honoraria; BMS Celgene: Honoraria; Abbvie: Honoraria; Jazz Pharmaceuticals: Honoraria; Daiichi-Sankyo: Honoraria; Astellas: Honoraria. Bertoli:Astellas: Honoraria. Heiblig:Astellas: Honoraria. Lambert:Astellas: Honoraria. Jourdan:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Carre:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Guieze:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie, Beigene, Janssen, Gilead, Roche, AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees. Laribi:AbbVie, AstraZeneca, Beigene, Iqone, Janssen, Novartis, Takeda: Honoraria. Pigneux:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Recher:Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.